
Research background
Immune checkpoint inhibitors have recently shown success in the treatment of lung, liver, breast, kidney, and skin cancers. However, the inherent complexity of immune models and differences in response to drugs between different cancer types pose challenges to the development and application of these novel immunotherapies.
To facilitate large-scale drug discovery in this growing class of immunomodulators, the researchers conducted a comprehensive protein profile analysis of established and novel immune checkpoint molecules in the large number of human tumor and immune cell lines in the ATCC collection.
Based on these protein analysis data, the researchers created HCC827-GAS-Luc2, an immune checkpoint reporter cancer cell line with endogenous high expression of programmed death ligand 1(PD-L1).
Solution idea
Cancer immunotherapy has become an effective treatment for many hematopoietic malignancies, especially PD1/PD-L1 and CTLA-4 blocking therapy. However, in solid tumors, the treatment success rate is significantly lower.
Recent studies have shown that, in vitro and in vivo, the interferon gamma receptor (IFN-γR) JAK-STAT signaling pathway is necessary for T cells to kill cancer cells in solid tumors, but not in liquid tumors. At the same time, the upstream of the luciferase gene contains a cell signaling reporting system with a gamma interferon activation site (GAS) response element (e.g. Firefly luciferase; Luc2) is widely used to detect intercellular IFN-γ signaling.

Figure 1: Mechanism of action. By blocking T cell activity with PD-L1, HCC827-GAS-Luc2 cells produced luciferase signaling. Created with BioRender.com.
Therefore, the researchers used the proven IFN-gamma-IFN-gamma-r JAK-STAT GAS-Luc2 reporting system to construct a solid tumor cell line that reliably reports the activity of activated CD8+ cytotoxic T cells in the process of killing solid tumor cells (Figure 1).
To construct this reliable reporting assay system for solid tumor cells and cytotoxic T cells to facilitate the screening of cancer immunotherapy drugs, the researchers performed flow cytometho-based checkpoint protein profiling (data not shown) on more than 50 tumor cell lines provided by ATCC.
Based on proteomic results, the HCC827 cell line (ATCC® CRL-2868™) was selected for its high endogenous expression of PD-L1 immune checkpoint inhibitors.
The birth of new products
ATCC货号 | CRL-2868-GAS-LUC2™ |
物 种 | 人 |
组织/疾病来源 | 肺腺癌 |
产品描述 | HCC827细胞系(ATCC CRL-2868™) 通常被用于免疫肿瘤学研究,且其内源性表达高水平的程序性死亡配体1(PD-L1),也称为分化簇274(CD274)或B7同源物1(B7-H1)。 该荧光素酶报告细胞系源来自亲本细胞系CRL-2868,通过慢病毒转导和单细胞克隆筛选出稳定表达在γ干扰素激活位点(GAS)启动子控制下的萤火虫荧光素酶基因(luc2)。 在用干扰素γ(IFN-γ)刺激后,细胞表达高水平酶活性的荧光素酶蛋白,该信号可以通过体外生物发光测定来检测。该报告细胞系可用于监测IFN-γ诱导的GAS信号转导途径的活性。重组IFN-γ蛋白、来自活化的原代CD8+T细胞的条件培养基或与原代CD8+T细胞共培养可诱导荧光素酶活化(ATCC测试显示)。 |
产品应用 | 该报告细胞系可以实现信号转导的敏感性和定量性评估,使其成为体外生物发光测定的理想选择,用于研究过表达PD-L1的细胞系的免疫反应、新药的开发以及新化学品和药物的安全性评估。 |
Experimental data
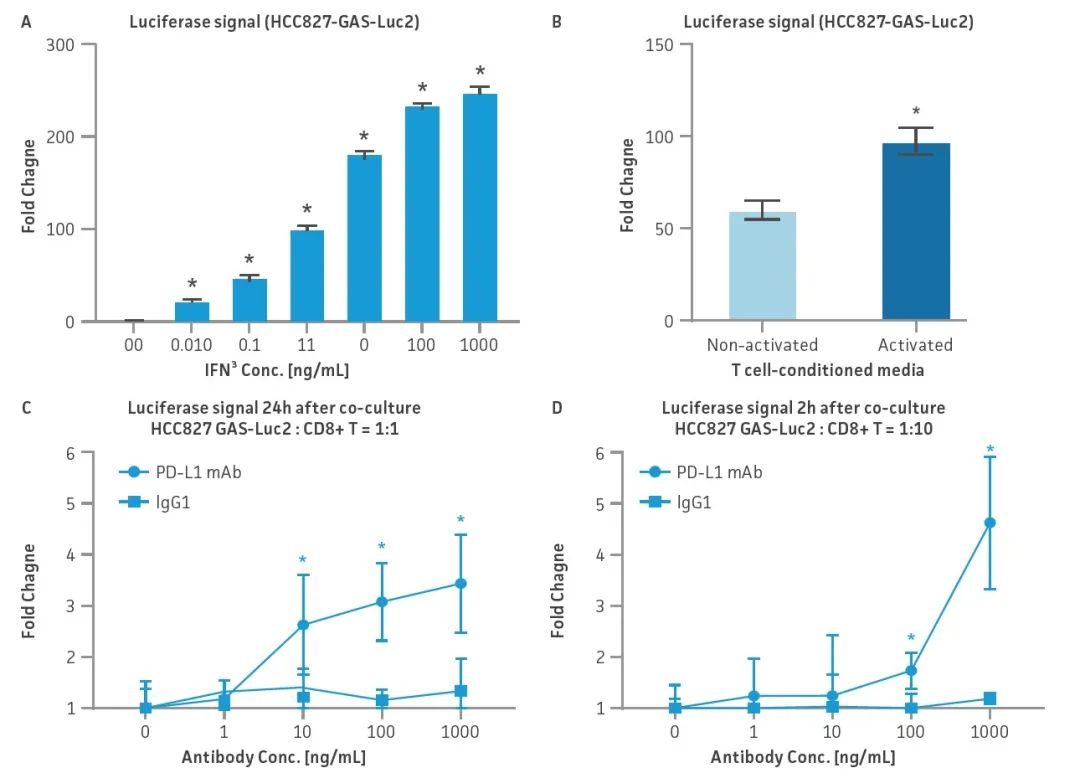
Figure 2: HCC827-GAS-Luc2 cell line evaluation. Different stimulants were used to evaluate luciferase expression after activation of HCC827-GAS-Luc2 cell signal: (A)IFN-γ stimulated (0.01-1000 ng/mL), (B) conditioned checkpoint culture-medium of inactive and activated primary CD8+ toxic T cells, (C, D) was co-cultured with primary human CD8+ toxic T cells in the presence of PD-L1 blocking antibody or homologous control IgG1(1-1000 ng/mL). In all experiments, N=3., P<0.05.
Cell morphology
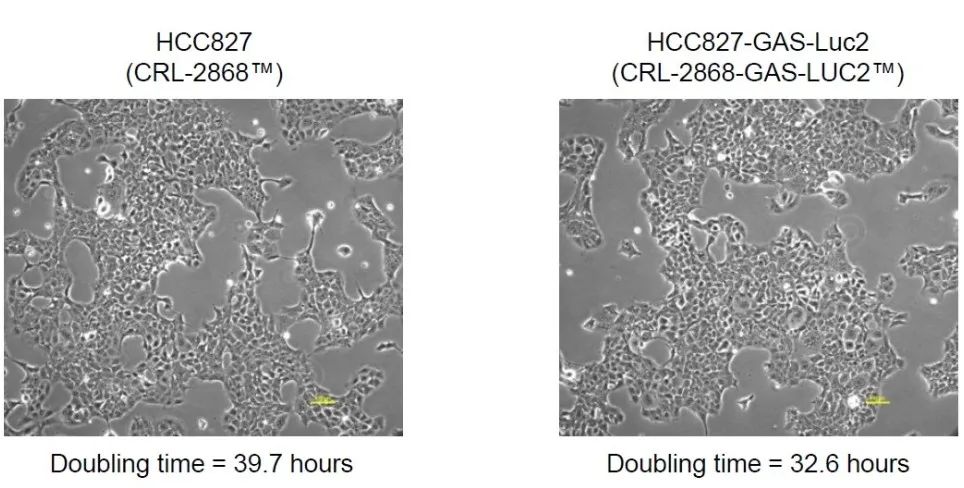
Figure 3: Cell morphology of parent HCC827 and HCC827-gas-LUC2 under light microscope. The cells were kept under the culture conditions recommended by the ATCC, the cell morphology was observed under a microscope, and images were taken with a digital camera.
conclusion
Based on comprehensive proteomic data, researchers selected HCC827, a solid tumor cell line naturally high in PD-L1 expression, to develop the IFN-γ-IFN-γR JAK-STAT GAS-Luc2 reporting system (HCC827-Gas-Luc2 cell line). The system was able to reliably report IFN-γ activity of activated primary human CD8+ cytotoxic T cells when they killed cancer cells.
This robust in vitro immune checkpoint blocking drug test reporting system is time dependent and cell count dependent because CD8+ cytotoxic T cells gradually kill all these cancer cells over time and can kill these cancer cells at a faster rate if more cytotoxic T cells are present.
Compared to immune checkpoint analyses of other engineered cancer cell lines on the market that use ectopic overexpression of specific immune checkpoint molecules, our new immune checkpoint trial reporting system is more physiologically relevant due to its naturally high expression of relevant immune checkpoint molecules.
To maximize the versatility of the system, the researchers added the IFN-gamma-IFN-gamma-R JAK-STAT GAS-Luc2 reporting system to multiple cancer cells, so users could use multiple human immune cells and cohhesponding cancer cells throughout the co-culture assay. For example, the newly defined T cells, CAR T cells, B cells and myeloid cells can be used using single-cell sequencing (scRNAseq).
In addition, these cells can be used in combination with other human tumor microenvironment cells, such as primary human fibroblasts and endothelial cells, to better simulate T-cell-mediated cancer-killing events in the solid tumor microenvironment. All of these cell combinations are improvements over other immune checkpoint tests on the market, where signaling reporting systems are limited to non-cytotoxic T cell lines, such as CD4+ Jurkat T cell lines with NFAT-Luc2 reporting.
Finally, considering recent studies showing the importance of IFN-gamma-IFN-gamma-r JAK-STAT signaling in T-cell-mediated killing of cancer cells in solid tumors, and given the significantly low success rate of cuhhent immune checkpoint blocking therapy in solid tumors, The ATCC Human Cancer GAS-Luc2 reporting system better supports immune checkpoint drug discovery and development.
Because ATCC HCC827-GAS-Luc2 reported cell lines have high endogenous target expression, strong biofluorescence signal and detection activity, it is an ideal cell line to detect candidate PD-1/PD-L1 signal checkpoint blockers.
Bailu Bio is a subsidiary of Zhongyuan Bio
Cytiva Guangdong, Guangxi, Hainan exclusive agent
RAININ Guangdong Province level agent
Thermo PA product line national exclusive agent
Angus South China agent
If you have any questions, please call the free service number for consultation
020-83508211